Views: 222 Author: Lake Publish Time: 2025-05-07 Origin: Site








Content Menu
● Introduction to Boron Carbide
● Understanding Boron Carbide's Electrical Conductivity
>>> Band Structure and Charge Transport
>> Temperature-Dependent Conductivity
>>> Low-Temperature Behavior (Below ~300 K)
>>> High-Temperature Behavior (Above ~300 K)
● Factors Influencing Electrical Conductivity
>> 1. Composition and Stoichiometry
>> 4. Microstructure and Porosity
● Applications Leveraging Boron Carbide's Electrical Properties
>> 1. Nuclear Reactor Control Rods
>> 2. High-Temperature Electronics
>> 3. Thermoelectric Materials
>> 4. Armor and Defense Systems
● Challenges and Research Directions
>> Material Purity and Synthesis
>> Understanding Defect Dynamics
>> Integration with Other Materials
>> Environmental and Economic Considerations
● Future Prospects and Innovations
>> Nanostructured Boron Carbide
>> Boron Carbide in Quantum Computing
● FAQ
>> 1. Is boron carbide a conductor or insulator?
>> 2. How does temperature affect boron carbide's conductivity?
>> 3. Can boron carbide be used in electronic devices?
>> 4. What role do defects play in its conductivity?
>> 5. Is boron carbide used in nuclear reactors?
Boron carbide (B₄C) is a remarkable ceramic material renowned for its extreme hardness, thermal stability, and neutron absorption capabilities. However, its electrical properties are less widely understood. This article explores whether boron carbide is a good electrical conductor, delving into its electrical conductivity mechanisms, temperature-dependent behavior, and applications that leverage its unique electronic characteristics. Through detailed explanations, visual aids, and practical insights, we aim to clarify the role of boron carbide in electrical and electronic contexts.

Boron carbide is a covalent compound composed of boron and carbon atoms arranged in a complex crystal structure. Its chemical formula is often approximated as B₄C, though its composition can vary within a homogeneity range (B₄C to B₁₀.5C). This material is prized for its exceptional hardness, ranking third after diamond and cubic boron nitride, and its resistance to harsh environments. Discovered in the 19th century, boron carbide has evolved from a laboratory curiosity to a critical material in defense, nuclear energy, and advanced manufacturing. Its unique combination of properties makes it a subject of ongoing research in materials science, particularly in understanding how its atomic structure influences functional behaviors like electrical conductivity.
- Hardness: ~9.5 on the Mohs scale.
- Density: 2.52 g/cm3.
- Melting Point: ~2,350°C.
- Thermal Conductivity: Moderate.
- Electrical Conductivity: Semiconductor behavior.
Boron carbide is classified as a semiconductor, meaning its electrical conductivity lies between that of conductors (e.g., metals) and insulators (e.g., ceramics like alumina). Its electronic properties are governed by a bandgap of approximately 2.09 eV, which determines how easily electrons can move between the valence and conduction bands. Unlike metals, where electrons flow freely, semiconductors require external energy (e.g., heat or light) to mobilize charge carriers. This property positions boron carbide as a candidate for specialized electronic applications where traditional semiconductors fail under extreme conditions.
The material's crystal structure-comprising B₁₂ icosahedra and C-B-C chains-creates localized electronic states. These structural units introduce irregularities in the electronic band structure, leading to a phenomenon known as electronic localization. Charge transport occurs through hopping mechanisms at low temperatures and thermal activation at high temperatures. In the hopping model, electrons "jump" between discrete energy states rather than moving freely, a process influenced by defects and stoichiometric variations.
At low temperatures, boron carbide exhibits variable-range hopping (VRH) conductivity, where electrons "hop" between localized states. This behavior follows Mott's law, indicating disordered electronic states within the bandgap. The hopping distance and energy depend on the density of defects and the material's stoichiometry. For example, carbon-deficient compositions introduce additional mid-gap states, enhancing hopping conductivity by providing more pathways for electron movement.
At elevated temperatures, conductivity becomes thermally activated. Electrons gain enough energy to overcome the bandgap or defect states, leading to increased conductivity. Activation energies correlate with the depth of defect states within the bandgap. This temperature dependence makes boron carbide suitable for sensors and devices operating in high-heat environments, such as industrial furnaces or aerospace components.
The boron-to-carbon ratio significantly impacts conductivity. Carbon-deficient compositions (e.g., B₁₀.5C) introduce structural defects that create mid-gap states, enhancing hopping conductivity. Excess boron, on the other hand, can lead to the formation of boron-rich phases, which may alter the material's electronic properties. Precise control of stoichiometry during synthesis is critical for tailoring conductivity for specific applications.
Disorder in the crystal lattice-such as vacancies, interstitial atoms, or substitutional impurities-generates localized electronic states. These states act as "stepping stones" for charge carriers, influencing hopping and thermal activation processes. For instance, boron vacancies can trap electrons, while carbon vacancies may introduce holes, modifying the overall conductivity. Advanced characterization techniques, such as positron annihilation spectroscopy, are used to study these defects.
Intentional doping (e.g., with silicon or nitrogen) or unintentional impurities (e.g., oxygen) can alter conductivity by modifying the density of states or introducing charge carriers. For example, silicon doping has been shown to reduce the activation energy for conduction, making the material more conductive at lower temperatures. However, impurities like oxygen can form insulating oxide layers, degrading performance in electronic applications.
Poorly sintered boron carbide with high porosity reduces effective conductivity due to interrupted electron pathways. Grain boundaries and voids act as scattering centers, impeding electron flow. Advanced sintering techniques, such as spark plasma sintering (SPS), produce denser microstructures with fewer defects, improving both mechanical and electrical properties.
Boron carbide's neutron absorption capability and moderate electrical conductivity make it suitable for control rods in nuclear reactors. Its conductivity aids in monitoring and control systems, enabling real-time adjustments to reactor operations. Additionally, its radiation resistance ensures long-term stability in high-flux environments.
As a semiconductor, boron carbide can operate in extreme environments (e.g., aerospace or industrial heating systems) where traditional silicon-based devices fail. For example, boron carbide sensors are used to monitor temperatures in jet engines, providing data without requiring cooling systems. Its wide bandgap also reduces leakage currents at high temperatures, enhancing device reliability.
Research explores boron carbide for thermoelectric applications, converting waste heat into electricity. Its thermal stability and tunable conductivity are advantageous. Recent studies focus on nanostructuring boron carbide to improve its thermoelectric figure of merit (ZT) by reducing thermal conductivity while maintaining electrical performance.
While primarily valued for mechanical properties, its electrical conductivity enables integration with sensors in smart armor systems. For instance, boron carbide panels embedded with strain gauges can detect impact locations and magnitudes, improving battlefield situational awareness. This dual functionality highlights its potential in next-generation protective gear.
Boron carbide's ability to interact with neutrons and its semiconductor properties make it a candidate for radiation detection devices. When exposed to neutron radiation, it generates charge carriers that can be measured to quantify radiation levels. This application is particularly relevant in nuclear safety and medical imaging.
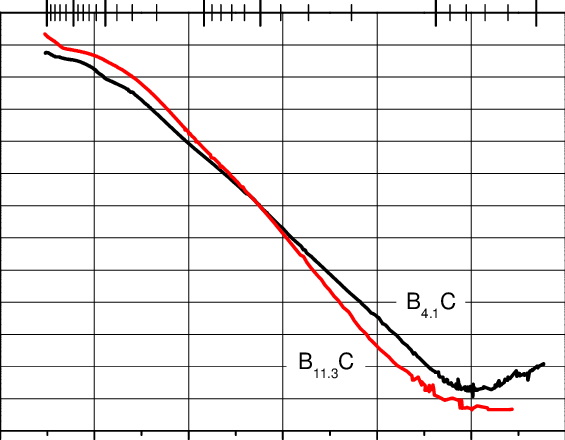
Achieving high-purity boron carbide with controlled stoichiometry remains challenging. Traditional synthesis methods, such as carbothermal reduction, often introduce impurities. Advanced techniques like chemical vapor deposition (CVD) and molecular beam epitaxy (MBE) offer better control but are cost-prohibitive for large-scale production. Research into scalable synthesis methods is critical for expanding its applications.
Ongoing studies focus on correlating specific defects (e.g., carbon vacancies) with electronic behavior to tailor conductivity for niche applications. For example, defect engineering could optimize boron carbide for use in photovoltaic cells or radiation-hardened electronics. Computational modeling, combined with experimental data, aids in predicting how defects alter electronic properties.
Combining boron carbide with conductive polymers or metals could enhance its utility in hybrid electronic systems. For instance, boron carbide-reinforced copper composites are being explored for use in high-wear electrical contacts. Similarly, boron carbide coatings on silicon substrates could improve the thermal and radiation resistance of semiconductor devices.
The production of boron carbide involves high energy consumption and generates waste, raising sustainability concerns. Research into recycling spent boron carbide abrasives or nuclear components could reduce environmental impact. Additionally, cost-effective synthesis methods are needed to make boron carbide viable for consumer electronics and renewable energy technologies.
Nanotechnology enables the fabrication of boron carbide with tailored properties. Nanowires and thin films exhibit enhanced electrical conductivity due to quantum confinement effects and reduced defect densities. These nanostructures are being tested in nanoelectromechanical systems (NEMS) and flexible electronics.
The unique electronic states in boron carbide's lattice make it a potential candidate for qubits in quantum computing. Its defect states could be manipulated to store quantum information, though this application is still in theoretical stages.
Boron carbide is not a good electrical conductor in the traditional sense but exhibits semiconducting behavior with unique charge transport mechanisms. Its electrical conductivity is highly dependent on temperature, composition, and defect structure. While unsuitable for high-current applications, its stability in extreme environments and tunable electronic properties make it valuable in specialized fields like nuclear energy, high-temperature electronics, and thermoelectrics. Future advancements in material synthesis, defect engineering, and hybrid material design could unlock new applications, from quantum computing to sustainable energy solutions. By addressing current challenges in purity and scalability, boron carbide may transition from a niche material to a cornerstone of advanced technologies.

Boron carbide is a semiconductor, with conductivity intermediate between metals and insulators.
At low temperatures, conductivity occurs via electron hopping; at high temperatures, thermal activation dominates.
Yes, its thermal stability makes it suitable for high-temperature electronics, though it is less efficient than silicon.
Defects create mid-gap states that facilitate hopping conductivity and influence thermal activation energies.
Yes, it serves in control rods due to neutron absorption and moderate electrical conductivity for monitoring systems.
Top Pure Silicon Carbide Manufacturers and Suppliers in Russia
Top Pure Silicon Carbide Manufacturers and Suppliers in France
Top Pure Silicon Carbide Manufacturers and Suppliers in Arabia
Top Polishing Silicon Carbide Manufacturers and Suppliers in Thailand
Top Polishing Silicon Carbide Manufacturers and Suppliers in Turkey
Top Polishing Silicon Carbide Manufacturers and Suppliers in Vietnam
Top Polishing Silicon Carbide Manufacturers and Suppliers in South Korea
Top Polishing Silicon Carbide Manufacturers and Suppliers in Japan
Top Polishing Silicon Carbide Manufacturers and Suppliers in Poland
Top Polishing Silicon Carbide Manufacturers and Suppliers in Portugal